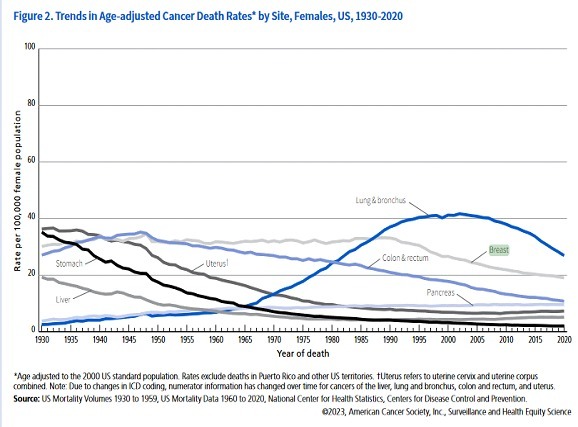
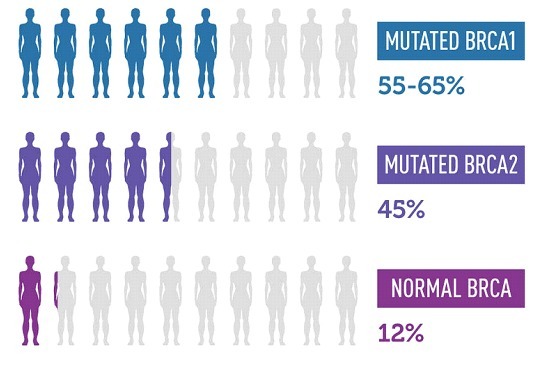
Empowering Health Decisions: How Genetic Testing Can Guide You
The Power of Genes: Exploring Genetic Testing Options
Genetic testing may be useful in determining whether an individual has a genetic condition or may develop one in the future.
Our body is made from millions of tiny cells. Each cell is controlled by thousands of genes. We have over 20,000 genes which are in every cell of our body and serve different functions.
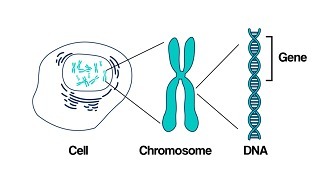
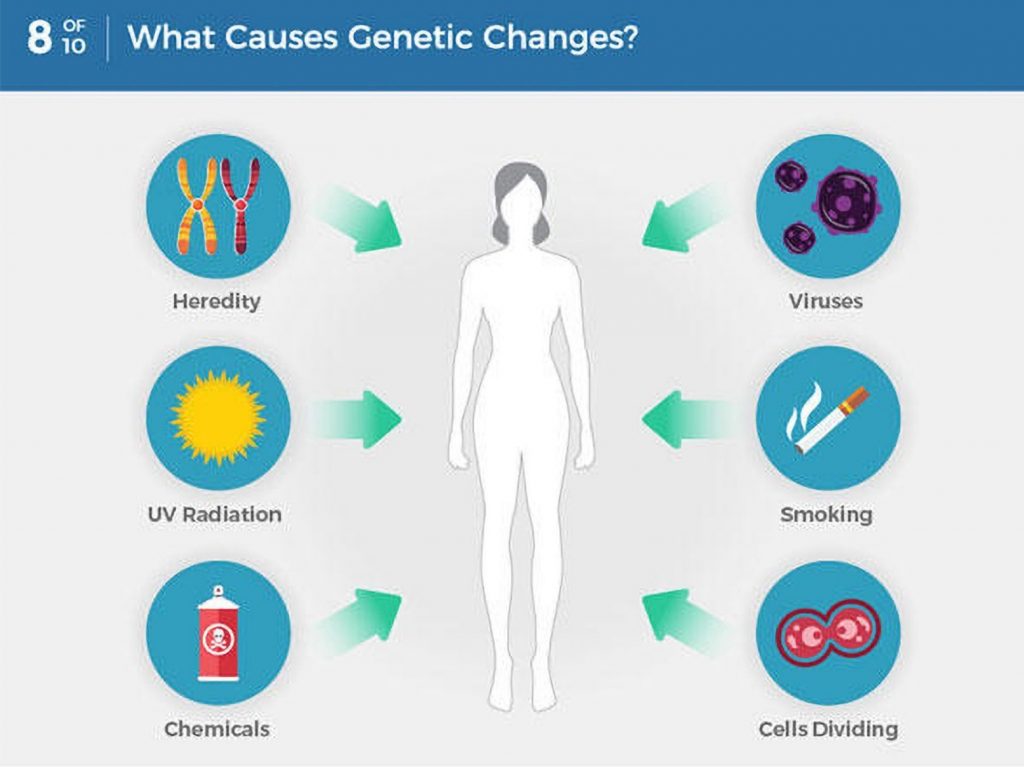
As we get older, we develop mutations within our genes – some are caused by outside factors like smoking or sun damage, while others happen at random. Genetic testing identifies changes in our genes or chromosomes, that are called mutations or variants.
Genetic testing has potential benefits, whether the results are positive or negative. Getting tested may:
- help you better understand your risk of cancer
- relieve your anxiety or uncertainty about disease risks like cancer, diabetes
- help you make decisions about your health and learn ways to help lower your risk of diseases
- help other family members decide if they should get tested or learn about how to lower their risk of certain diseases
- lead to finding cancer earlier when treatment is most likely to work better
When it comes to health and disease — and, of course, many other aspects of life — one thing is certain: genes matter.
Types of Genetic Tests and Why Are They Important?
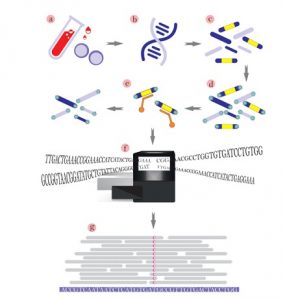
There are many kinds of genetic tests. Limitations to that, there is no single genetic test that can detect all genetic conditions.
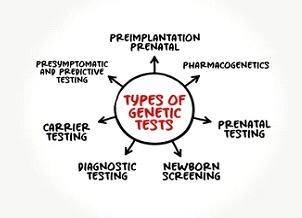
The approach to genetic testing is individualized based on your medical and family history and what condition you’re being tested for.
Single gene testing. Single-gene tests look for changes in only one gene. Single-gene testing is done when your doctor believes you or your child have symptoms of a specific condition or syndrome that is well-established for single-gene disorder. Some examples of this are Duchene muscular dystrophy and sickle cell disease. Single-gene testing is also used when there is a known genetic mutation running in a family.
Panel testing. A panel genetic test looks for changes in many genes in one test. Genetic testing panels are usually grouped into categories based on different kinds of medical concerns. Some examples of genetic panel tests are low muscle tone, short stature, or epilepsy. Panel genetic tests can also be grouped into genes that are all associated with a higher risk of developing certain kinds of cancer, like breast or colorectal cancer, etc.
Large-scale genetic or genomic testing. There are two different kinds of large-scale genetic tests.
- Exome sequencing looks at all the genes in the DNA (whole exome) or just the genes that are related to medical conditions (clinical exome).
- Genome sequencing is the largest genetic test and looks at all of a person’s DNA, not just the genes.
Exome and genome sequencing are ordered by doctors for people with complex medical histories. Large-scale genomic testing is also used in research to learn more about the genetic causes of conditions.
Comparison and benefits of Gene testing:
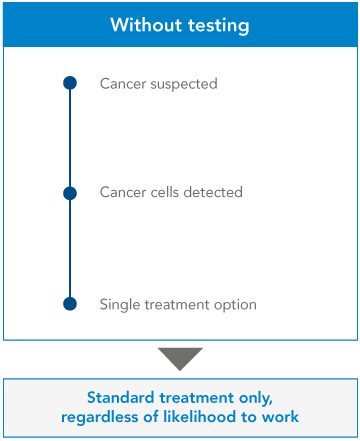
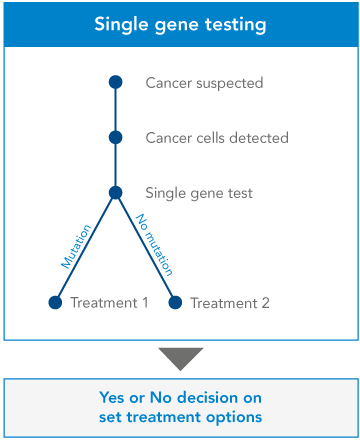
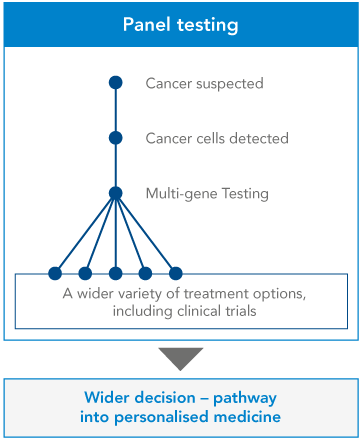
Genetic testing is the key to understanding our genes, providing a window into our past, guiding our present health decisions, and illuminating a path toward a healthier future.